ACCELERATE – Five years accelerating cancer drug development for children and adolescents
Improving survival and reducing long-term side-effects for children and adolescents with cancer is the key priority for ACCELERATE, since the number of new oncology drugs in paediatric development remains too low and the improvement in the survival rates for childhood malignancies has plateaued.
ACCELERATE’s latest scientific manuscript highlights all the changes in the landscape of paediatric oncology, the platform’s achievements over the past years, the challenges remaining, and future directions to advance the innovation of therapies for children and adolescents with cancer.
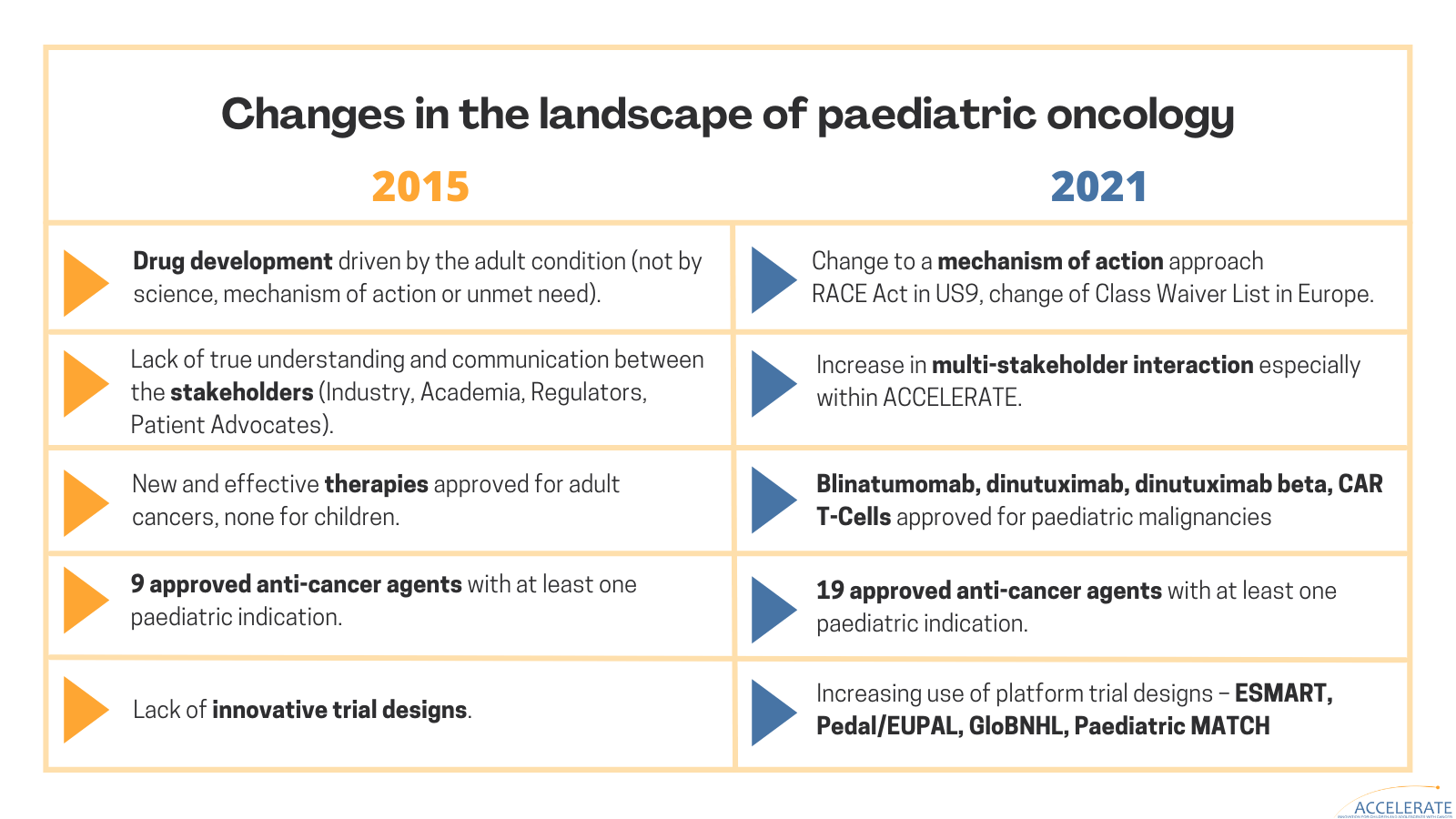
Early engagement between all stakeholders in development of new drugs is critical. ACCELERATE has enhanced communication and understanding between academia, industry, patient advocates and regulators, advanced the timely investigation of new anti-cancer drugs, and promoted mechanism-of-action driven drug development approach.
What are ACCELERATE’s goals?
- Prioritisation of medical products and a focused and sequential strategy.
- Early assessment of promising drugs in adolescents through their inclusion in adult early phase trials.
- Support alignment in paediatric cancer drug development plans between the European Medicines Agency and the US Food and Drug Administration.
- Identify unmet medical needs through international multi-stakeholder collaboration.
The 7 key results of ACCELERATE’s projects
Mechanism of Action drug development
The first Working Group created by ACCELERATE was “Mechanism of action (MOA) driven drug development”. Their work centred on the fact that drug development for children and adolescents with cancer should follow a mechanism of action-based approach rather than being driven by the adult condition.
Paediatric Strategy Forums
Paediatric Strategy Forums are multi-stakeholder meetings in which strategies regarding new drug development are discussed. Each Forum focuses on either a given paediatric malignancy or the development of a class of compounds with regards to their mechanism of action. These scientific meetings are aimed at facilitating prioritisation in order to better meet the needs of patients and to increase feasibility of paediatric developments.
ACCELERATE has organized nice Forums focusing on different compounds: ALK inhibition, Mature B-cell lymphoma, CheckPoint Inhibitors, Acute Myeloid Leukemia, Epigenetic modifiers, BET inhibitors, Second ALK inhibition, CAR T cells, mTKI in Bone Sarcomas, and MAPK pathway inhibitors. More than 350 experts from all around the world have attended the forums.
Inclusion of adolescents and Young Adults (AYA) in adult trials
The FAIR Group (Fostering Age Inclusive Research) was established in 2017 by 15 experts from Academia, Industry and Parents/Patients advocacy. It aims to improve access for adolescents to new anti-cancer drugs and to make the drug development process more efficient
The Group has developed a consensus article endorsed by regulatory bodies and industry. There are ongoing actions to raise awareness to the professionals involved in trial design and approval as well as the general public.
Fit for Filing
The scope of Fit for Filling (FFF) Working Group is to develop best principles on how to design and deliver an academic or academic and industry collaborative trial with a dataset that can be included in a package for regulatory filing.
The Group aims to develop a model for investigator-initiated clinical trials of new drugs to meet the regulatory requirements through the “Fit for Filing”. An educational programme to promote this approach is also currently under development and will be delivered in 2022.
Strengthening global collaboration
International collaboration in clinical trials is essential to develop new and better drugs for children and adolescents with cancer. The International Collaboration Working Group has created a data survey of intercontinental trials to identify obstacles and believes that a multi-stakeholder discussion can build consensus to identify solutions. Further, the systematic review “Intercontinental collaboration in clinical trials for children and adolescents with cancer” was published in October 2021.
Long-term follow up
The vision of the Long Term Follow Up (LTFU) Working Group is to create an international, open, harmonised and sustainable data registry to collect long term side effects of new anti-cancer therapies in children. This registry will help to fulfill the regulatory requirements of the marketing authorisation holders and to provide knowledge of the long term safety and follow up care of new modalities to support the best use of these therapies while providing important information for patients and families.
What is next?
Developing new drugs for children with cancer is a global endeavour and ACCELERATE envisions to expand the initiatives to become increasingly global. The platform will remain the perfect setting to enhance dialogue, collaboration, understanding, and transparency among all stakeholders.




